Lead Carbonate And Sulfuric Acid Equation . Pb2+(aq) + 2cl−(aq) → pbcl2(s) p b 2 + (a q) + 2 c l − (a q) → p b c l 2 (s) adding. the chemical equation is shown below: The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for. 2ch3ch2co2h(aq) + ca(oh)2(aq) → (ch3ch2co2)2ca(aq) + 2h2o(l) the reaction of a. learn how to write and balance chemical equations in different forms, such as molecular, complete ionic, and net ionic. Enter an equation of an ionic chemical equation and press the balance button. the balanced chemical equation is as follows: english as a second language (speaking endorsement) past papers. The balanced equation will be.
from www.thesciencehive.co.uk
english as a second language (speaking endorsement) past papers. 2ch3ch2co2h(aq) + ca(oh)2(aq) → (ch3ch2co2)2ca(aq) + 2h2o(l) the reaction of a. The balanced equation will be. Enter an equation of an ionic chemical equation and press the balance button. the chemical equation is shown below: Pb2+(aq) + 2cl−(aq) → pbcl2(s) p b 2 + (a q) + 2 c l − (a q) → p b c l 2 (s) adding. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for. the balanced chemical equation is as follows: learn how to write and balance chemical equations in different forms, such as molecular, complete ionic, and net ionic.
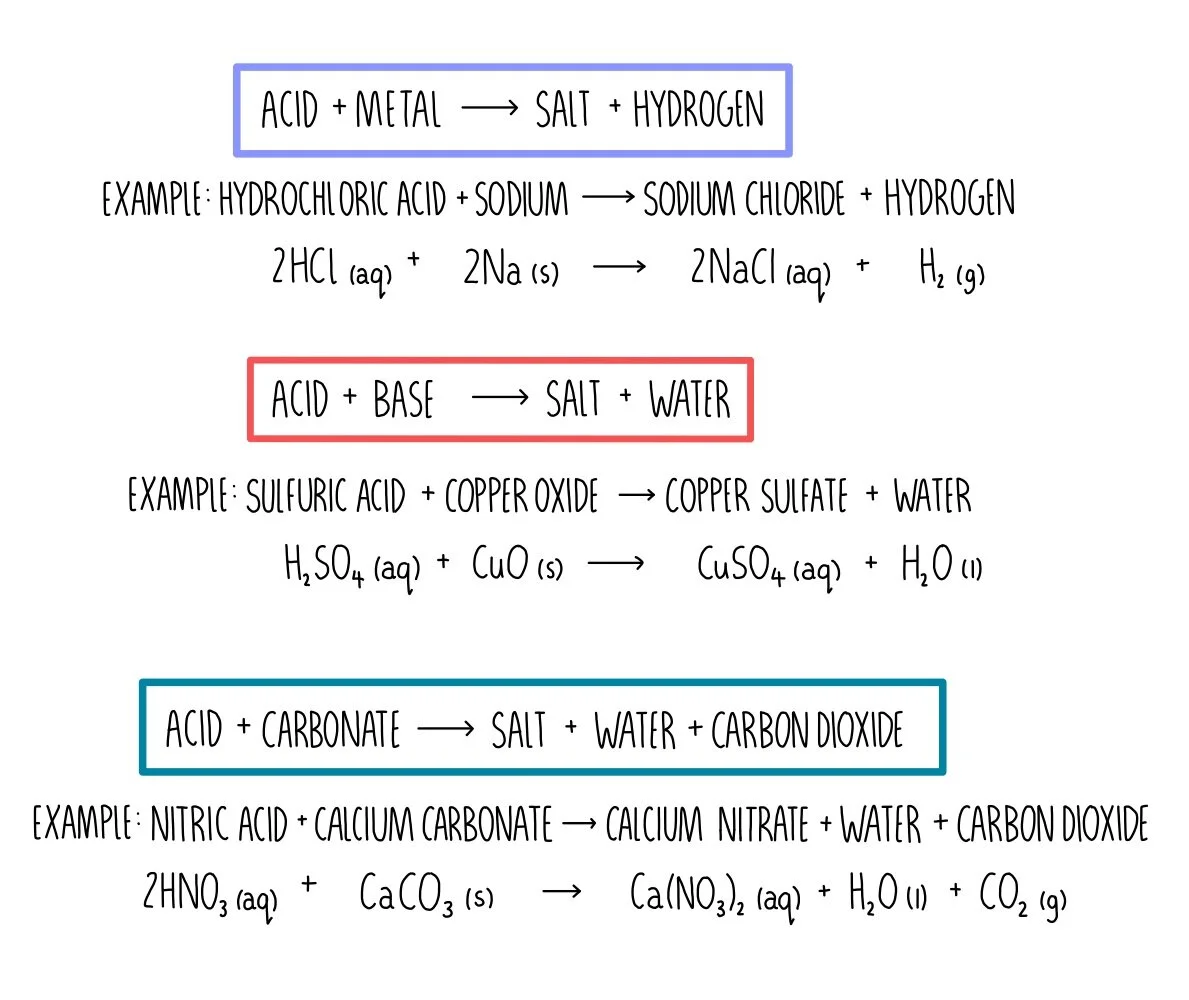
Acids, Bases and Salt Preparations (GCSE) — the science hive
Lead Carbonate And Sulfuric Acid Equation Enter an equation of an ionic chemical equation and press the balance button. Enter an equation of an ionic chemical equation and press the balance button. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for. the chemical equation is shown below: learn how to write and balance chemical equations in different forms, such as molecular, complete ionic, and net ionic. The balanced equation will be. Pb2+(aq) + 2cl−(aq) → pbcl2(s) p b 2 + (a q) + 2 c l − (a q) → p b c l 2 (s) adding. english as a second language (speaking endorsement) past papers. 2ch3ch2co2h(aq) + ca(oh)2(aq) → (ch3ch2co2)2ca(aq) + 2h2o(l) the reaction of a. the balanced chemical equation is as follows:
From aliananewsgaines.blogspot.com
What Is Formed When Calcium Carbonate Reacts With Sulfuric Acid Lead Carbonate And Sulfuric Acid Equation the balanced chemical equation is as follows: learn how to write and balance chemical equations in different forms, such as molecular, complete ionic, and net ionic. Pb2+(aq) + 2cl−(aq) → pbcl2(s) p b 2 + (a q) + 2 c l − (a q) → p b c l 2 (s) adding. The balanced equation will be. The. Lead Carbonate And Sulfuric Acid Equation.
From aliananewsgaines.blogspot.com
What Is Formed When Calcium Carbonate Reacts With Sulfuric Acid Lead Carbonate And Sulfuric Acid Equation the balanced chemical equation is as follows: learn how to write and balance chemical equations in different forms, such as molecular, complete ionic, and net ionic. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation. Lead Carbonate And Sulfuric Acid Equation.
From www.animationoptions.com
Sulfuric Acid Structure Lead Carbonate And Sulfuric Acid Equation learn how to write and balance chemical equations in different forms, such as molecular, complete ionic, and net ionic. Pb2+(aq) + 2cl−(aq) → pbcl2(s) p b 2 + (a q) + 2 c l − (a q) → p b c l 2 (s) adding. The balanced equation will be. The chemical equation described in section 4.1 is balanced,. Lead Carbonate And Sulfuric Acid Equation.
From www.numerade.com
SOLVEDWrite an equation to show how sulfuric acids in acid rain reacts Lead Carbonate And Sulfuric Acid Equation The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for. The balanced equation will be. learn how to write and balance chemical equations in different forms, such as molecular, complete ionic, and net ionic. Enter an equation of an ionic chemical equation and press the balance button. the balanced chemical equation is. Lead Carbonate And Sulfuric Acid Equation.
From tia-jolpblogramsey.blogspot.com
Zinc Carbonate Sulfuric Acid Lead Carbonate And Sulfuric Acid Equation the balanced chemical equation is as follows: the chemical equation is shown below: 2ch3ch2co2h(aq) + ca(oh)2(aq) → (ch3ch2co2)2ca(aq) + 2h2o(l) the reaction of a. learn how to write and balance chemical equations in different forms, such as molecular, complete ionic, and net ionic. english as a second language (speaking endorsement) past papers. The balanced equation will. Lead Carbonate And Sulfuric Acid Equation.
From exouyldhc.blob.core.windows.net
Lead Carbonate + Sulphuric Acid at Harold Petersen blog Lead Carbonate And Sulfuric Acid Equation the balanced chemical equation is as follows: Enter an equation of an ionic chemical equation and press the balance button. Pb2+(aq) + 2cl−(aq) → pbcl2(s) p b 2 + (a q) + 2 c l − (a q) → p b c l 2 (s) adding. the chemical equation is shown below: 2ch3ch2co2h(aq) + ca(oh)2(aq) → (ch3ch2co2)2ca(aq) +. Lead Carbonate And Sulfuric Acid Equation.
From www.chegg.com
Solved 1) Select the net ionic equation for the reaction Lead Carbonate And Sulfuric Acid Equation Enter an equation of an ionic chemical equation and press the balance button. the chemical equation is shown below: The balanced equation will be. english as a second language (speaking endorsement) past papers. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for. 2ch3ch2co2h(aq) + ca(oh)2(aq) → (ch3ch2co2)2ca(aq) + 2h2o(l) the reaction. Lead Carbonate And Sulfuric Acid Equation.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Lead Carbonate And Sulfuric Acid Equation the chemical equation is shown below: english as a second language (speaking endorsement) past papers. Pb2+(aq) + 2cl−(aq) → pbcl2(s) p b 2 + (a q) + 2 c l − (a q) → p b c l 2 (s) adding. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for. . Lead Carbonate And Sulfuric Acid Equation.
From www.numerade.com
SOLVED Sodium carbonate and sulfuric acid Molecular Equation Complete Lead Carbonate And Sulfuric Acid Equation the balanced chemical equation is as follows: Pb2+(aq) + 2cl−(aq) → pbcl2(s) p b 2 + (a q) + 2 c l − (a q) → p b c l 2 (s) adding. english as a second language (speaking endorsement) past papers. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for.. Lead Carbonate And Sulfuric Acid Equation.
From www.numerade.com
⏩SOLVEDSolutions of sulfuric acid and lead(II) acetate react to Lead Carbonate And Sulfuric Acid Equation english as a second language (speaking endorsement) past papers. the chemical equation is shown below: Pb2+(aq) + 2cl−(aq) → pbcl2(s) p b 2 + (a q) + 2 c l − (a q) → p b c l 2 (s) adding. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will. Lead Carbonate And Sulfuric Acid Equation.
From www.numerade.com
SOLVED Write the balanced equation for the reaction of sodium Lead Carbonate And Sulfuric Acid Equation english as a second language (speaking endorsement) past papers. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be. Pb2+(aq) + 2cl−(aq) → pbcl2(s) p b 2 + (a q) + 2 c l − (a q) → p b c l 2 (s) adding. the chemical equation is shown. Lead Carbonate And Sulfuric Acid Equation.
From www.numerade.com
Lead (II) carbonate to form lead (II) oxide and carbon Lead Carbonate And Sulfuric Acid Equation 2ch3ch2co2h(aq) + ca(oh)2(aq) → (ch3ch2co2)2ca(aq) + 2h2o(l) the reaction of a. the balanced chemical equation is as follows: learn how to write and balance chemical equations in different forms, such as molecular, complete ionic, and net ionic. the chemical equation is shown below: Pb2+(aq) + 2cl−(aq) → pbcl2(s) p b 2 + (a q) + 2 c. Lead Carbonate And Sulfuric Acid Equation.
From www.numerade.com
SOLVED The preparation of lead sulphate from lead carbonate is a two Lead Carbonate And Sulfuric Acid Equation The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for. english as a second language (speaking endorsement) past papers. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be. the balanced chemical equation is as follows: the chemical equation is shown below: . Lead Carbonate And Sulfuric Acid Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Na2S + Zn(NO3)2 = NaNO3 + ZnS Lead Carbonate And Sulfuric Acid Equation Enter an equation of an ionic chemical equation and press the balance button. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for. the balanced chemical equation is as follows: the chemical equation is shown below: 2ch3ch2co2h(aq) + ca(oh)2(aq) → (ch3ch2co2)2ca(aq) + 2h2o(l) the reaction of a. learn how to write. Lead Carbonate And Sulfuric Acid Equation.
From www.thoughtco.com
10 Common Acids and Chemical Structures Lead Carbonate And Sulfuric Acid Equation The balanced equation will be. Enter an equation of an ionic chemical equation and press the balance button. 2ch3ch2co2h(aq) + ca(oh)2(aq) → (ch3ch2co2)2ca(aq) + 2h2o(l) the reaction of a. Pb2+(aq) + 2cl−(aq) → pbcl2(s) p b 2 + (a q) + 2 c l − (a q) → p b c l 2 (s) adding. learn how to write. Lead Carbonate And Sulfuric Acid Equation.
From www.numerade.com
SOLVED Sodium carbonate and sulfuric acid Molecular Equation Complete Lead Carbonate And Sulfuric Acid Equation the chemical equation is shown below: the balanced chemical equation is as follows: The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for. learn how to write and balance chemical equations in different forms, such as molecular, complete ionic, and net ionic. Enter an equation of an ionic chemical equation and. Lead Carbonate And Sulfuric Acid Equation.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Lead Carbonate And Sulfuric Acid Equation english as a second language (speaking endorsement) past papers. the chemical equation is shown below: learn how to write and balance chemical equations in different forms, such as molecular, complete ionic, and net ionic. the balanced chemical equation is as follows: The balanced equation will be. Enter an equation of an ionic chemical equation and press. Lead Carbonate And Sulfuric Acid Equation.
From www.youtube.com
How to write the equation for PbCO3 + H2O Lead (II) carbonate + Water Lead Carbonate And Sulfuric Acid Equation Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be. Pb2+(aq) + 2cl−(aq) → pbcl2(s) p b 2 + (a q) + 2 c l − (a q) → p b c l 2 (s) adding. the chemical equation is shown below: the balanced chemical equation is as follows: The. Lead Carbonate And Sulfuric Acid Equation.